Myelofibrosis is one of the Philadelphia chromosome-negative myeloproliferative neoplasms (MPN) characterized by clonal myeloproliferation. Allogeneic hematopoietic stem-cell transplantation is the only potentially curative treatment for patients with myelofibrosis. With that, it can resolve fibrosis and lead to complete loss of the clonal burden. More than 90% of myelofibrosis patients harbor driver mutations in one of the 3 genes JAK2, CALR, and MPL, with JAK2 mutations being associated with worse outcomes.
We herein report a study to determine whether the persistence of cells with MPN-associated driver mutations in the early period after allogeneic hematopoietic stem-cell transplantation was associated with outcomes. Patient samples for molecular analysis were prospectively collected from peripheral blood at 5 different time points: at start of conditioning prior to stem cell infusion, at days 30, 100, 180, and 1 year after transplantation. We applied quantitative PCR technology to detect individual driver mutations in JAK2, CALR, and MPL (with a high sensitivity of 0.01%). Simulation was applied to explain variances at different time points. Multivariable modelling was employed to determine independent effects.
A total of 312 patients receiving first reduced-intensity conditioning transplantation were included. Distribution of driver mutation genotype at time of transplantation was as follows: 67% with JAK2, 19% with CALR, 4% with MPL, and 8% were triple-negative. Conditioning regimen comprised busulfan and fludarabine. Donor platform for transplantation was: matched related (18%), matched unrelated (58%), haploidentical (1%), and mismatched unrelated (23%). Disease risks at time of transplantation were low (1%), intermediate-1 (22%), intermediate-2 (55%), and high (22%). Median follow-up of the total cohort was 6 years.
The median allele burden at time of transplantation was significantly (P<0.001) different between driver mutations: 20% (range, 0.1-100%) for JAK2, 44% (range, 3-54%) for CALR, 60% (range, 43-99%) for MPL.
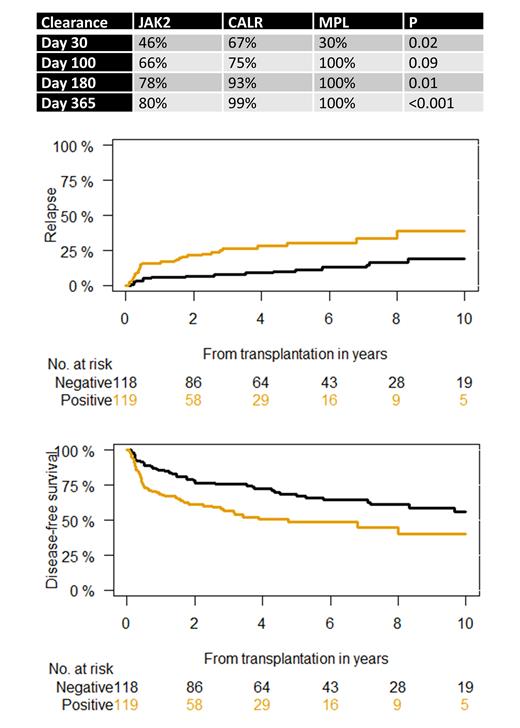
CALR and MPL showed earlier mutation clearance after transplantation compared with JAK2 ( Table). Mutation clearance was achieved at day 30 after transplantation in 46% with JAK2, 67% for CALR, and 30% for MPL (P=0.02). At day 100 after transplantation, 66% of JAK2, 75% of CALR, and 100% of MPL showed complete mutation clearance (P=0.09).
Complete mutation clearance at day 30 after transplantation was predictive of post-transplantation relapse at 1 year (P=0.009), showing cumulative incidence of relapse of 6% versus 16%. Similar results were seen for complete mutation clearance at day 100 after transplantation, demonstrating cumulative incidence of relapse of 4% versus 24% (P<0.001). Mutation clearance at day 30 and 100 after transplantation explained 80% and 87% of variance of later time points. The effect of mutation clearance on post-transplant relapse was irrespective of driver mutation status ( Figure).
6-year disease-free survival according to mutation type was 52% for JAK2, 63% for CALR, and 80% for MPL (P=0.045). Mutation clearance at day 30 after transplantation was associated with better disease-free survival, irrespective of driver mutation status ( Figure). Moreover, day-30 complete mutation clearance predicted disease-free survival in JAK2, showing rates of 60% for negative versus 47% in JAK2 positive patients (P=0.05). Importantly, achievement of mutation clearance at day 30 after transplantation could also distinguish different risk groups in patients with onset better prognosis harboring CALR/MPL mutations. The 6-year disease-free survival was 76% for patients with mutation clearance versus 59% for CALR/MPL-positive patients(P=0.02) .
Multivariate analysis (adjusting for donor type, diagnosis of primary or secondary myelofibrosis, allele burden at time of transplantation, and age) confirmed that patients who had mutation clearance at day 30 after transplantation had lower risk of relapse, better disease-free and overall survival.
In conclusion, this is the first study to show that CALR and MPL are associated with earlier mutation clearance after transplantation compared with JAK2. Mutation clearance at day 30 and 100 after transplantation was the best predictor of relapse and disease-free survival, independent of driver mutation status.
Disclosures
Kroeger:Novartis, Neovii: Honoraria, Research Funding; Celgene/BMS: Honoraria, Research Funding; AOP Orphan Pharmaceutical, JAZZ, Takeda, Astellas: Honoraria; Riemser: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal